USA TODAY, Consumer Reports -- Conspirators in mass Fraud and Deception
By Jack Owoc CEO, CSO VPX Sports
This article is written in response to the recent attacks by USA Today and Consumer Reports (links are posted above) on the dietary supplement industry and an ingredient called AMP. Their unfiltered behavior is a threat to the freedom of all Americans to have access to cutting edge dietary supplements.
In a bitter twist of irony, not once did VPX receive a call from the co-conspiring purposeful purveyors of mass fraud and deception on the public at large concerning the naturally occurring AMP (2-Amino-4-Methylpentane) compound. The recent press is referring to this compound as DMBA to make it sound like DMAA simply to cause unjustified controversy. For the record nobody in the dietary supplement industry uses the questionable and confusing DMBA nomenclature except in USA Today and Consumer Reports. This is just one of many acts of fear mongering, fact twisting and deception by USA Today and Consumer Reports that we will point out in this article. In discussions about AMP nomenclature it should be noted that chemically speaking, 2-Amino-4-Methylpentane, 4-methyl-2-pentylamine, and 4- Amino-2-Methylpentane are the exact same compound. There is no difference between the three naturally occurring compound names and therefore, they can be used interchangeably when referring to AMP. From here on out we will call the naturally occurring green tea compound by its real name, AMP and will refuse to placate to the tomfoolery inflicted on all of us by the recent rash of irresponsible journalism and media events launched by USA Today and Consumer Reports.
AMP has been ingested for thousands of years in both Pouchung and Green Tea
AMP or 2-Amino-4-Methylpentane is naturally occurring in the food supply via the fermentation process of both Pouchung Tea and Green Tea. Pouchung Tea has been ingested for thousands of years. In the latter part of this document we provide irrefutable sophisticated scientific testing that proves that AMP is a naturally occurring compound in Pouchung Tea.
*To review the testing procedures and methodologies please see the bold highlighted paragraph titled: Irrefutable proof that AMP aka 2-Amino-4-Methylpentane is found in the food chain and is 100% DSHEA compliant: near the end of this article.
The landmark Dietary Supplement Health and Education Act of 1994 (DSHEA)
DSHEA protects consumers’ rights to readily have access to supplements (1), and The National Health Freedom Action calls DSHEA a "foundational cornerstone of health freedom in our country”. (2)
Yet DSHEA is being chipped away and relentlessly attacked since the act was made a law 20 years ago.
On October 25 1994, president , Bill Clinton signed the DSHEA Act into law, saying that "After several years of intense efforts, manufacturers, experts in nutrition, and legislators, acting in a conscientious alliance with consumers at the grassroots level, have moved successfully to bring common sense to the treatment of dietary supplements under regulation and law.” (3)
Yet, DSHEA Authors -- Senator Hatch and Senator Harkin are disrespected and their interpretation of the law they wrote is disregarded and trampled upon.
So how does DSHEA, written by Senator’s Hatch and Harkin, define a Dietary Supplement?
DSHEA defines the term "dietary supplement" to mean a product (other than tobacco) intended to supplement the diet that bears or contains one or more of the following dietary ingredients: a vitamin, a mineral, an herb or other botanical, an amino acid, a dietary substance for use by man to supplement the diet by increasing the total dietary intake, or a concentrate, metabolite, constituent, extract, or combination of any of the aforementioned ingredients. Furthermore, a dietary supplement must be labeled as a dietary supplement and be intended for ingestion and must not be represented for use as conventional food or as a sole item of a meal or of the diet. In addition, a dietary supplement cannot be approved or authorized for investigation as a new drug, antibiotic, or biologic, unless it was marketed as a food or a dietary supplement before such approval or authorization. Under DSHEA, dietary supplements are deemed to be food, except for purposes of the drug definition. (4)
AMP Found in most Widely Consumed Beverage on Planet Earth
Even though AMP is a 100% natural constituent of a species of Green Tea and Pouchung Tea and has been in our food supply for thousands of years, USA Today claims it is “untested on humans”. This would be no different than saying chlorogenic acid which is found in coffee has never been tested on humans when 500 billion cups were consumed in 2010 alone.(5) The fact is that consumers are drinking millions of metric tons of tea annually and it’s a major part of our global economy. http://www.slideshare.net/subby123/global-tea-production-consumption-trends-32386463 (6)
Therefore, it’s safe and appropriate to say that USA Today’s statement that AMP “is a new chemical stimulant – untested on humans” is an untrue statement meant to garner unmerited media sensationalism at the expense of the public at large. In a desperate attempt to remain relevant where social media is now the major go-to outlet for authentic “consumer reports”, ConsumerReports.org even goes one step falsely stating, “MD2 Meltdown” (a product I recently developed containing AMP) “and other supplements were found to contain a dangerous drug.” So now, according to Consumer Reports, a naturally occurring ingredient found in Green Tea is a “dangerous drug”. Ironically, they claim AMP is “untested on humans”, yet claim it’s “a dangerous stimulant” and even “a dangerous drug”, yet by their own admission it is “untested in humans” and offer absolutely no truth to substantiate these outlandish claims. It’s worth repeating: The facts are that AMP has been in the food supply and consumed by humans for thousands of years.
VPX Supplements -- Backed by 20 University Double Blind Placebo Controlled Gold Standard Studies
Keep in mind that the VPX line of products that I developed are backed by an unprecedented 20 University Studies including but not limited to: UCLA, Baylor University, University of Memphis, Florida State University, University of Southern Maine and many others by the most renowned research scientists in the America. For these reasons the slanderous and defamatory remarks by Pieter Cohen, USA Today and Consumer Reports are highly disconcerting.

VPX Meltdown® – backed by 5 Double Blind Placebo Controlled Gold Standard University Studies
The previous version of Meltdown (prior to the release of the new MD2 Meltdown) was backed by five university studies. The fat blasting study results of Meltdown were so profound that prominent researchers couldn’t believe the data and went over the math at least a half a dozen times. Meltdown has been sold for over 15 years and to the best of my knowledge there has never been even one single adverse event reported during this time! Again I repeat-- For these reasons the slanderous and defamatory remarks by Pieter Cohen, USA Today and Consumer Reports are highly disconcerting.

The After the Fact Attack
It wasn't until after the AMP article already hit news wires nationwide did our phones start blowing up for comment. If these news outlets wanted the truth, they would have attempted to call VPX for comment before nationally publishing a barrage of scathing lies and deception. No attempt to call me or my company for comment came until after the articles hit the press. Yet, these organizations and individuals spent a great deal of time planning their attack and unlawfully securing and positing unauthorized, defamatory images of VPX products next to unapproved stock model images. By claiming they attempted to call us, USA Today and Consumer Reports make no mention that they purposefully did not contact.
Consumer Reports Fails to Conduct due Diligence
In this same article Consumer Reports falsely accuses VPX of marketing to adolescents. Yet, VPX is the very company who championed a national effort in schools to prevent marketing to energy drinks to kids! In fact, VPX publicly announced its $25,000 offer to the Broward County School System to put a program in place to educate minors not to consume energy drinks. VPX was applauded by TV news stations for displaying four warnings on its packaging stating, NOT INTENDED FOR USE BY INDIVIDUALS UNDER THE AGE OF 18. By failing to conduct research, Consumer Reports unfairly disparages VPX/Redline by posting a product called Redline White Heat that states in all caps: NOT INTENDED FOR USE BY INDIVIDUALS UNDER THE AGE OF 18 right on the label.
This is yet another extreme example of unjust enrichment by Consumer Reports to profit from ill-gotten gain at the expense of the very "consumer" they claim to be protecting.
Scientists launch a subversive, self-serving bogus assault on ingredient found in the most consumed beverage on Planet Earth -Tea.
So why attack AMP? I believe it’s a case of obscure individuals and the dying print media outlets desperately attempting to remain relevant. When you’ve become obsolete and irrelevant you have to scream sensational absurdities louder and louder.
Follow the Money
The hip and sometimes highly profitable thing to do if you’re a PhD or MD is to attack stimulants with unscientific and dramatic claims. A 140 pound nerd can safely attack a 280 pound pre-workout-using athlete from behind his computer screen and shred him to pieces. On the other hand, the willingness of a PhD or MD to compromise authentic science in exchange for sensationalistic hype then results in the media continually contacting the PhD or MD for more sensationalized negative commentary regarding supplements as we all witnessed in the case with Pieter Cohen who is quoted in the USA Today and Consumer Reports articles:
After a PhD or MD garners enough attention in the media, he or she is then positioned as a so-called “expert” who attorneys can count on to give false testimony on the stands similar to what the PhD or MD does in the media. This creates an unfair advantage in class action and other suits where attorneys and law firms are enriched to the tune of millions of dollars and the class (consumer) gets $10 if he or she is willing complete, obtain and fill out a claim form and endure further hassle to get the refund. These are high stakes cases where PhD and MD experts can charge in excess of $600/hour and sometimes rack up bills over $100,000! It results in a massive win/win for the media outlet, the PhD or MD and the law firm, but at the extreme expense of small businesses and consumers at large. At the end you lose as supplement prices skyrocket. You can bet experts were waiting to testify that Red Bull does or doesn’t (depending who is paying the expert) “Give You Wings”! You see the “expert” always sides with the firm who is paying him or her. Here’s a prime example of a very recent lawsuit where attorneys will get millions and consumers will get close to nothing.
Red Bull Settles Lawsuit for $13M Because the Drink Doesn’t Really ‘Give You Wings’
If a lie is repeated enough among critics of authentic science, that lie is then perceived as truth! Pieter Cohen specializes in crafting junk science while despising and being an outspoken critic of DSHEA and real science.
Cohen calls current FDA approach “woefully inadequate” and scaremongers that it may have “life threatening consequences”. (8) I often wonder if the MD acronym following Cohen’s name stands for Master of Drama? Because if the “MD” stands for medical doctor, why doesn’t Cohen ever go after Big Pharma whose “medically prescribed” drugs will kill and debilitate more persons in the next five minutes than in the 30 year history of the ultra-safe dietary supplement industry? Please tell us Cohen – we are literally “dying” to know?
It's a strange case of the tail trying to wag the dog -- various irrelevant persons and organizations are uttering disrespect toward the FDA. Therefore, I would like to commend the FDA for not giving in and placating to all the abusive commentary directed at them by Cohen et al.
These carefully crafted articles by USA Today and Consumer Reports use a series of disingenuous double standard attacks on the sports nutrition industry. If it’s a fat burner or pre-workout product, the media and its supporting cast of characters launch an all out attack. Redline White Heat pictured below is a pre-workout product that contains AMP.

“For this study Cohen and researchers from the Netherlands and NSF International, an independent testing organization, looked for products that listed something called "AMP" or "AMP citrate" on the label because they had not previously seen it in supplements and wondered what it might actually be. They discovered that it was chemically similar to the banned stimulant DMAA. DMBA showed up in 12 of the 14 samples of the supplements they tested. What's even more disconcerting, the scientists say, is that the number of supplements containing DMBA is probably much greater.”
Good job Cohen and the NSF – you actually proved that VPX products stringently meet label claim!
“You remove a few atoms from the chemical structure of a stimulant and you’re exposing adolescents and adults to completely novel drugs," Cohen said. "It’s the equivalent of purchasing a research chemical and swallowing it. We have no idea what is going to happen to you.”
Here Cohen’s babbles scientifically unsound absurdities that are grossly lacking in coherence and cohesion. Further, Cohen’s statements are libelous, slanderous, disingenuous, and damaging. So I ask, “Cohen -- Who removed atoms from a chemical structure?” Other than trying to deceive the public at large and the fact that you know nothing about chemistry, what are you trying to accomplish here with your outrageous gibberish and balderdash? All VPX products mentioned clearly state: “WARNING: NOT BY USE FOR INDIVIDUALS UNDER THE AGE OF 18.” IN ALL BOLD CAPS PROMINENTLY ON THE LABEL. Therefore, I ask –who is selling to adolescents? You has neither the qualifications nor the right to publically mouth off and disparage the most university gold standard studied product line in the history of the dietary supplement industry!
“Given the potential health risks, Cohen says the FDA should warn consumers about supplements containing DMBA, and that manufacturers need to recall those products.”
I’m sure everybody is wondering and so I will ask: Cohen who and/or what gives you the authority and audacity to habitually tell the FDA what they should and should not be doing?
“When Consumer Reports contacted the maker of Redline White Heat and MD2 Meltdown, VPX Sports Nutrition Supplement Company of Weston, Fla., for comment, a company spokeswoman said it was not interested in speaking to us.”
Consumer Reports never tried to contact myself nor anybody at VPX until after the deceptive AMP articles were secretly launched. This shoot-first-and-ask- questions-later was and is a subversive and reprehensible tactic by the media.
The deadly chemical methamphetamine known as “Crystal Meth has a nearly-identical chemical structures to b-phenylethylamine (b-PEA). However, b-PEA is a harmless and health-promoting dietary supplement responsible for the mood elevating effects of chocolate. b-PEA is found extensively in nature and is naturally occurring in chocolate, many plant species and a myriad of other natural organisms The compound bPEA also known as “the love compound” exerts a very positive effect on mood etc. However, because of scare mongering tactics similar to that carried out by the USA TODAY, Consumer Reports et al, the entire Smoothie King chain has now pulled the highly beneficial and super safe b-PEA ingredient from their shelves nationwide. Once again we have media-fueled purposeful misinformation and disinformation at the expense of the consumer’s health and well being undermining DSHEA and the constitutional rights of all Americans. This further illustrates the unscientific argument and utter nonsense that similar chemical structures have similar effects in the body and puts to rest the unsubstantiated argument that DMAA is similar to AMP!
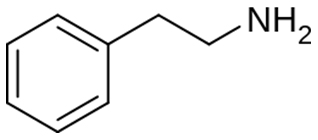
Chemical structure of Methamphetamine AKA Crystal Meth
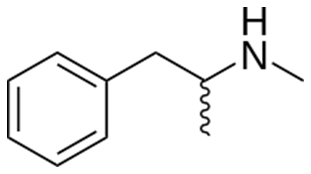
According to the no-science nonsense regarding similar chemical structures being one and the same -- the media is claiming that this:

Is the same as this:
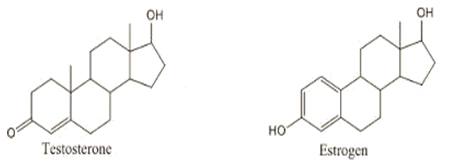
The “chemical cousins” argument debunked! While chocolate contains bPEA which has a chemical structure similar to Crystal Meth, they are NOT even remotely alike. Likewise, testosterone and estrogen are not alike and neither is DMAA and AMP alike!

Look at me – Are you going to play the “chemical cousin card” and tell me PEA is the same as Crystal Meth or that Testosterone is the same as Estrogen? Seriously? Then don’t even try to say that AMP is the same as DMAA!
The Scientific Truth and AMP
AMP or 1,3-dimethylbutylamine, or as it will be referred to here, DMBA has come under fire as the result of a new study titled “A synthetic stimulant never tested in humans, 1,3 dimethylbutylamine (DMBA), is identified in multiple dietary supplements .” The title of the study is somewhat misleading, as the products examined did not need any identification – each listed the ingredient in question on the bottle. Those unfamiliar with supplement labeling guidelines might see this title and conclude that these researchers found an unlisted or undeclared ingredient, when in fact, the opposite is true.
Inclusion criteria for the study was that the supplements were labeled to contain the ingredient in question, and that they were available from a US distributor in April of 2014. Immediately the inclusion criteria was disregarded as one product was ordered from the United Kingdom and not available in the United States. No explanation is given for the immediate failure of the scientists to accurately follow their own exclusion criteria.
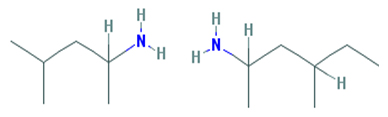
The paper itself begins with a reference to DMBA being an analogue of DMAA (1,3 dimethylamylamine), and as proof, a diagram of the two compounds side by side:
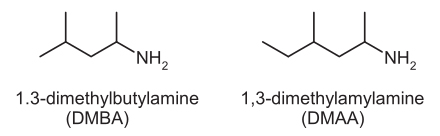
However, in a two dimensional rendering, many things with opposite biological effects can look similar. Here's a look at testosterone versus estrogen, polar opposites:
We find the rendering used to illustrate the similarities between DMAA and DMBA deceptive in its simplicity (the more simple we make a chemical diagram, the more similar they will appear). Here's how the National Institute of Health renders DMBA and DMAA (following the format above, DMAA appears on the right):
The dissimilarities are even more pronounced if we were to use a three dimensional rendering of DMAA versus DMBA (again, taken from the NIH):
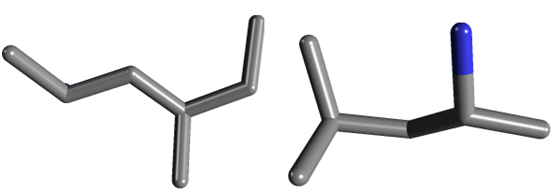
Of course, the world is not two dimensional, and when we see these compounds rendered as they are in 3D, it's fairly obvious that there are several notable differences. More, in fact, than we see with testosterone versus estrogen.
Unfortunately, besides the claim that DMBA is an analogue of DMAA, and the picture that follows, no proof was offered to support the claim. Again, looking at the records found within the National Institute of Health, two compounds are listed as having a 3D structure related to DMAA and ten with related bioactivities, and none of them are DMBA. Therefore, without any data to support the claim that DMBA is an analogue of DMAA, and the failure of any supporting authority to make the same claim, it must be dismissed in its entirety as false.
The article at hand also seems to cast aspersions on two other papers that discovered DMBA in the food supply (in tea – the most widely consumed beverage in the world - and a plant, respectively), where no reference standard was used. It bears noting that the same authors collaborated on a paper, published eleven months prior, that purported to find an undeclared ingredient in a dietary supplement, for which they also used no reference standard. That prior paper was later found to be highly inaccurate, as noted in the latest issue of the AHPA Report (The Official Publication of the American Herbal Products Association).
However, the point remains that it is unclear why the authors of the current work require a reference standard from their colleagues, while being under no obligation to provide one themselves.
The gravitas of this situation becomes compounded when the circumstances surrounding the publication of this paper are exposed fully. Prior to the work being released publicly, it was released privately to journalists, in an attempt to gain media coverage for the work. Subsequently, journalists (who can not be blamed for lacking the scientific acumen to validate the work) were able to publish articles in the mainstream media, before the industry was given a chance to review the paper – which, as should be obvious by now – is sorely lacking in validity.
None of this is surprising, as the media-hungry anti-supplement stance of the lead author, Dr. Cohen, has been well documented through his multiple appearances on The Dr.Oz show and elsewhere.
Further compounding the situation is the attempts by both the authors and the media to call governmental attention to the paper. This is distressing because the authors display their lack of understanding as regards the regulatory framework of dietary supplements. This is highlighted by their observation that the DMBA found in supplements is likely to be synthetic because even small amounts would require a metric ton of natural material to produce, which is presented as support for the argument that it should be classified as a drug. The authors go so far as to state that this fact makes it “...very difficult, if not impossible...” to classify DMBA as a dietary supplement.” However, extraction from a natural source is not a requirement for a dietary supplement to be compliant with the relevant laws – otherwise, a bottle of vitamin C (assuming 500 capsules of 1,000mgs) would require more than 5,000 oranges to produce. In this case, the argument is rendered invalid because the conclusion does not follow from any of the premises.
Hence, we have seen this paper widely disseminated by a media who understandably lacks the training required to evaluate its validity, much less understand DSHEA, and further thrust into the political spectrum by those who have repeatedly shown ignorance of the very federal laws and guidelines they seek to change. It is also unclear why, in making regulatory recommendations for the United States, they rely on a reference from the European Journal of Food Research & Review on DMAA, which deals with European laws, which neither deals with the laws nor compound in question. We praise the FDA for not adding needless regulation or classification, especially not at the word of Dr.Oz's supplement expert.
Re-classifying an ingredient or rewriting legislature needs to follow the proper legal and scientific guidelines. It is therefore contradictory that the paper states that there is no human data on DMBA, but simultaneously calls for its classification as a pressor-amine, which by definition requires that exact data. It is worth noting that in the absence of human data, animal research can give insight to the presumed actions and effects of a compound in humans. For this reason, the authors support their pressor-amine argument by referencing a study that they claim provides DMBA data in animals (performed in 1944 by Rohrmann and Shonle). Upon review, that study suggests DMBA to be a far safer substance than DMAA.
We're also told that the World Anti-Doping Agency will likely ban DMBA because of it's similarity with tuamine, and are subsequently provided a reference that only mentions the latter compound, and does not mention DMBA at all, much less it's alleged similarity (Thevis et. Al, 2007). This is not how references work- they need to support the preceding thought. In this way, many references in this paper fail, as does the paper itself.
The media (Consumer Reports) has claimed that we, at VPX Sports, are marketing these products to teens, ignoring the fact that have been featured nationally, both in print and television, as championing the cause of keeping these types of products out of the hands of teenagers. We also have 20 gold standard double blind placebo controlled studies on our finished products, including 30 markers of human subject blood safety, in a product used for four continuous weeks. None of those facts will sell magazines, however, and they were conveniently ignored.
While the paper erroneously cites numerous references that fail to be relevant, it makes no attempt to call to attention our commitment to science and safety, and as a result, neither has the media. In this light it's difficult to view either as seeking the truth on this matter.
Unfortunately, we have scientists claiming that no data exists, providing dubious references, and holding their peers to a standard they have failed to maintain. They are calling for a revision of laws that they lack a basic understanding of, and their primary weapon is not science or facts, but rather press releases and the manipulation of our media.
Science does not work that way, and thankfully, neither do the laws.
Irrefutable proof that AMP aka 2-Amino-4-Methylpentane is found in the food chain in Pouchung Tea and is 100% DSHEA compliant:
Analysis of mass spectrometry identified a total of 29 species, of which 3 - exthoxylpropanal, thiobis methane, dimethyl sulfide, acetic acid ethye ester, 2-hexanone and content of trans-2-hexenal and other ingredients with storage time of growth and decline, while propionaldehyde, 1-penten-3-ol, n-capronaldehyde, acetic acid, pentanal, 2,5-hexane-dione, 4-methyl-2-pentanamine (AMP) and The 2,4-hephadinal content increased.
Description: Pouchung teas made of Chin-shin Oolong and Taiwan Tea-12 (TTES-12) stored at -20, 25 and 45degreeC for 6 months were used as experimental materials. The purposes of this study were to identify their volatile components and to investigate their changes during storage. Their volatile components were identified using a Headspace Sampler/Gas Chromatograph/Mass Spectrometer. The changes in volatile components were examined every 3 months. At the same time, the aroma intensities and overall preference of these Pouchung teas were also evaluated by 6 well-trained and 30 consumer-type panelists, respectively. Totally, 40 volatile components in these two Pouchung teas were identified. Among them, dimethyl sulfide was found to be the most abundant one with amounts of 7.42 and 5.78 ppm in the Chin-shin Oolong and TTES-12 teas, respectively. During storage, components in tea stored at -20degreeC did not change significantly. For those samples stored at 25 and 45 degree, the contents of 4-6 components, e.g., dimethyl sulfide, ethyl acetate, 2-hexanone and trans-2-hexenal decreased while those of 5-6 components, e.g., propionaldehyde, n-capronaldehyde, and acetic acid increased as the storage time increased. Pentanal, with 2,4-heptadeinal in Chin-shin Oolong tea and 2,5-hexanedione with 4-methyl-2-pentylamine (4-methyl-2-pentanamine is 2-amino-4-methylpentane aka AMP) in TTES-12 tea appeared after storage for one month and then increased during storage. Correlation coefficients for the aroma intensity and overall preference scores of the two Pouchung teas with the total change in contents of volatile components during storage were all around 0.80. The correlation coefficients between the aroma intensity and overall preference scores of the Chin-shin Oolong and TTES-12 teas were 0.83 and 0.87, respectively.

This study is in two phases. In the first phase, a variety of tea samples and Pouchung tea were analyzed by near infrared spectrometer on their Chemical compositions, and sensory quality analysis was also done. The second phase investigated the effects of different sample preparations on the quality of Pouchong tea’s storage period. Seventy kinds of tea were analyzed via NIR for their chemical components excluding fat contents (moisture content, total nitrogen, total polyphenols, and color). The R-value of the calibration curve was greater than 0.92, the measured and predicted values of the correlation coefficient (r) were higher than 0.96. Regarding to the chemical compositions of Pouchong tea, each component of the calibration curve of the R-value was all above 0.95(except the R-value of color of Green Heart oolong and Jin Xuan tea were 0.91 and 0.93), both the predicted and measured values of the correlation coefficient (r) were higher than 0.96. Amino acid analysis showed that some of the amino acids with higher levels of content and range are more likely to get a high R-value, such as
Aspartic acid, Serine, Proline, Valine, and Tryptophan. From the analytical results of Pouchung tea’s functional quality by NIR, C.S. Oolong tea and TTES-12 are very close, the correlation coefficient values (r) of appearance and color are approximately 0.80. The aroma components are in second level which is bad smell with r-value at 0.75 (produced during storage), whereas first level is good smell with r-value at 0.85 (The special aroma from fresh tea). The r-value for taste is 0.88. After 6 months storage at different packaging and storage temperature, C.S. Oolong tea and TTES-12 were taken out to test the contents of moisture, Vitamin C, polyphenols, catechin, and fatty acids, as well as color and sensory evaluation. The C.S. Oolong tea and TTES-12 in 4 different packaging materials bottles (OPP/CPP, KOP/PE/EVA, PE, PET), and in different oxygen content (0%, 10%, 20%), and in at different temperature (-20C, 5C, 25C, and 45C), and with or without exposure to light, after 6 months, the changes on chemical compositions and favorability were analyzed. The results: By comparison the samples in PET and KOP/PE/EVA bottles with nitrogen filled (0% oxygen) to Pouchung tea at room temperature, and those in OPP/CPP (or PE) normal packaging (20% oxygen), and those at -20C and 5C, the changes of contents of vitamin C, catechin, and fatty acids are close. There were no significant difference between two samples, but the shelf life of the former was longer. The volatiles of Pouchung tea were alalyzed by GC/MS, and found 29 chemical components totally. The contents of 3-Exthoxylpropanal, thiobismethane, dimethyl sulfide, acetic acid ethye ester, 2-hexanone and trans-2-hexenal decreased with the storage. Whereas, the contents of propionaldehyde, 1-penten-3-ol, n-capronaldehyde, acetic acid, pentanal , 2,5-hexane-dione, 4-methyl-2-pentanamine (2-amino-4-methylpentane aka AMP), and 2,4-hephadinal increased.
Here is a summary of the above:
They used the NIR in the first part of the study to determine the correlation between the different types of teas- with certain software on the NIR and a pretty big sample size for each type of tea (which will account for variation), you can do a mathematical equation that shows a correlation between the different varieties- it basically puts a numerical value on the similarities between the different types of tea. It does provide some documentation that this compound can be found in the food chain occurring as a food (this is the way DSHEA words it) in certain types of tea. For example, D-amino acids aren’t normally naturally-occurring, however, they manifest themselves during cooking, acidification, and other food prep processes, so that makes them acceptable to use from a DSHEA compliance standpoint, because they are normally present in the food chain due to normal processing procedures.
In conclusion – AMP or DMBA is a naturally occurring constituent contained in the most consumed beverage on planet Earth which is tea. AMP is proven to be found in the Pouchung Tea by the extensive testing as previously described. AMP has been consumed in the food supply by humans for thousands of years and is 100% DSHEA compliant and legal.
Thank you for reading this response to the USA Today and Consumer Reports attack on our outstanding dietary supplement industry and DSHEA-compliant AMP. If you find this article helpful please pass it along to others and continue to fight with me to preserve free access to cutting edge and affordable dietary supplements. Do not allow anyone to put a lid on science and prevent our access to the countless God-given ingredients contained in botanicals around the world.
Sincerely,
Jack Owoc CEO, CSO VPX Sports and promoter of optimal health and performance for all.
1) http://www.slideshare.net/subby123/global-tea-production-consumption-trends-32386463
2) Alliance for Natural Health- USA. "Dietary Supplement Health and Education Act (DSHEA)."
3) Jump up! National Health Freedom Action. "Protect DSHEA!"
4) http://www.health.gov/dietsupp/ch1.htm
5) International Coffee Organization (ICO). Statistics. Breakdown of exports of green Arabica and green. Robusta of countries exporting significant volumes of both types of coffee June 2009, January 2011. www.ico.org (accessed January 21, 2011)
6) http://www.slideshare.net/subby123/global-tea-production-consumption-trends-32386463 (6)
7) Janet Rehnquist Department of Health and Human Services - Office of the Inspector General - Dietary Supplement Labels: Key Elements, United States Department of Health and Human Services
8) Cohen PA. Hazards of Hindsight – Monitoring the Safety of Nutritional Supplements. N Engl J Med 2014;370:1277-1280.








